Efferon LPS receives CE mark approval in the European Union as a class 2b medical device under MDR requirements.
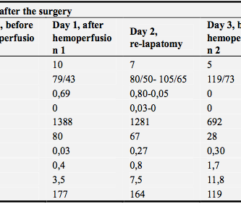
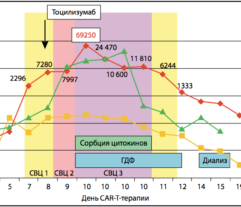
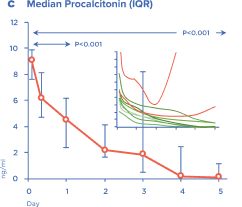
Efferon LPS is a single-use adsorbent-filled cartridge for extracorporeal blood purification. Patients’s blood is perfused through the adsorbent and is returned into bloodstream. Tailored surface and structure of adsorbent beads bind bacterial endotoxins and excessive inflammatory mediators (such as cytokines) and remove then from the circulation.
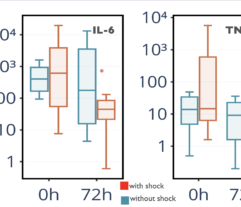
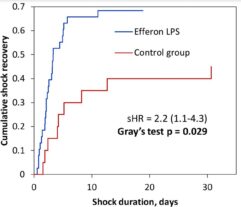
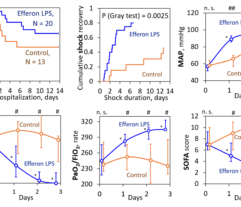
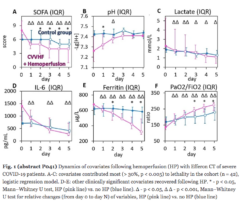
Multicenter randomised clinical study [Shock. 2023 Jun 1;59(6):846-854] validated safety and efficacy of Efferon LPS in patients with septic shock. This treatment improved their vital functions, reduced duration of multiple organ deficiency and increased survival rate.
We congratulate physicians, patients and their families who are gaining long awaited access to an innovative extracorporeal treatment of sepsis, septic shock and multiple organ failure syndrome.
Sepsis is a deadly foe and now we will fight it together.