Results of LASSO multicentered randomised clinical trial are published in Shock journal.
Patients with abdominal sepsis, complicated with septic shock, were enrolled into study (n=58) and randomised in two groups (2:1). Both study groups were treated using standard of care, the first group was additionally treated with Efferon LPS. Initial 12 hours after onset of septic shock (requirement of infusion of vasopressor drugs to maintain blood pressure) were a time window for the intervention.
- Main conclusions:
The use of hemoperfusion with Efferon LPS was associated with significant improvement in patients with septic shock.
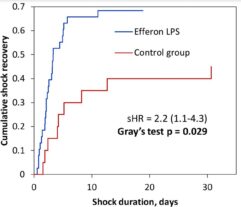
1. Hemodynamic parameters: increase in mean arterial pressure and decrease in the need for high-dose norepinephrine (both after 24 h) and dramatically shorter shock duration among survivors (57 h vs. 101 h, respectively).
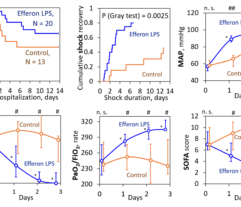
2. Respiratory function: lesser duration of MV among the survivors (2.6 vs 4.8 days), increased cumulative MV weaning and an increase PaO2/FiO2 ratio (starting 24 h post-EHP).
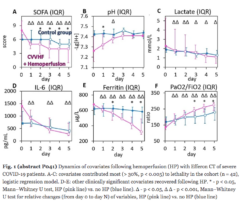
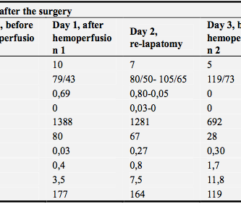
3. Renal function: Decreased serum creatinine levels and reduced RRT requirements.
4. Multiple organ deficiency severity decreased starting 24 h post-EHP as evidenced by decreasing the SOFA score.
5. Decreased levels of inflammatory markers PCT, CRP, IL-6, leukocyte, and neutrophil counts were accompanied by a reduction in bacterial LPS levels in 75% of patients.
Statistically significant improvements in vital parameters were accompanied by trends in the successful resolution of septic shock (68% vs. 45% in the control group at 72 h) and increased 3-days hospital survival vs. the control group (87% and 60%, respectively, p=0.012). Trend toward decreased hospital mortality, including 28-day mortality, in the Efferon LPS group compared with the control group was not significant (47% vs. 55%, p=0.783) and warrant new trials with increased statistical power.
Full text is available at: https://doi.org/10.1097/SHK.0000000000002121