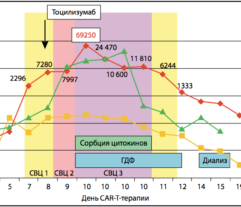
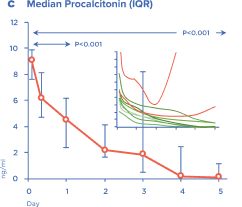
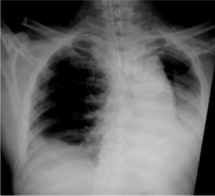
LASSO (lipopolysaccharide adsorption during septic shock) the first multicenter randomized clinical trial of Efferon LPS devices has been launched. Research centers – leading Moscow clinics: city clinical hospital № 1 named after N.I. Pirogov, City Clinical Hospital № 68 named after V.P. Demikhova, city clinical hospital named after S.S. Yudin, Research Institute of Ambulance named after N.V. Sklifosovsky. The first patients to receive therapy with Efferon LPS have already been recruited into the study.
The research is conducted by the independent CRO-company Ligand Research, the independent laboratory service Exacte Labs takes part in the work.
LASSO is planned and conducted in accordance with the requirements of good clinical practice and ethical principles outlined in ISO 14155. The study protocol is open and available on the international clinical trial registration platform – clinicaltrials.gov.
S.I. Rey, Ph.D., Senior Researcher in Research Institute of Ambulance named after N.V. Sklifosovsky:
“Every day, resuscitators in their practice are faced with gram-negative sepsis and septic shock, so the possibility of elimination of endotoxin applying extracorporeal hemocorrection procedures is of considerable interest. Conducting a randomized controlled trial LASSO will help us assess the effectiveness and safety of this procedure and, if positive results are obtained, use this technique on the territory of the Russian Federation in an extremely difficult contingent of patients.”
I.V. Bessonov, technical director and co-founder of JSC Efferon:
“Our doctors are the most talented, experienced and knowledgeable. In Russia, it is possible and necessary to conduct advanced research at the world level. With the LASSO project, we want to set a new standard for transparency and evidence. ”
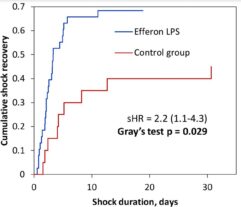
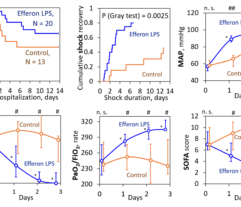
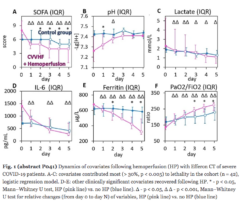
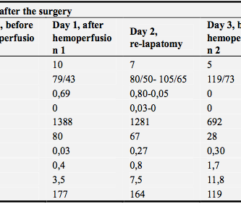
Efferon LPS is a single-use medical device for selective hemosorption of lipopolysaccharides and excess the first in its class, used in the intensive care of sepsis and septic shock.